The Quiet Squeeze on 8‑Year‑Plus Whiskey
Why mature liquid remains scarce even as younger barrels soften — and what it means for buyers For the last two years, the dominant narrative in bulk...
2 min read
 Matt Breese
:
Jul 10, 2025 11:33:57 AM
Matt Breese
:
Jul 10, 2025 11:33:57 AM
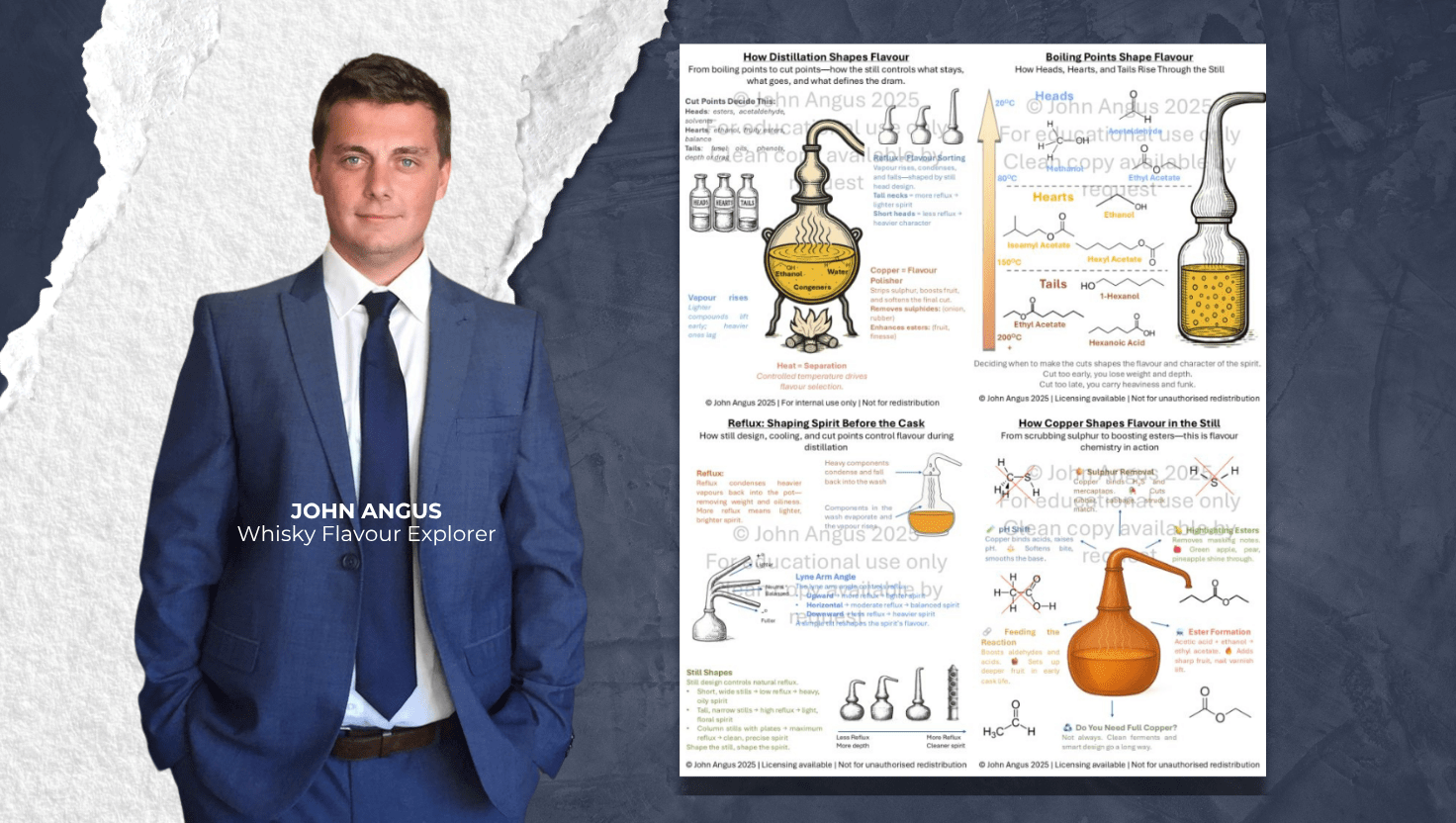
Distillers are often seen as artists—but underneath the surface, they’re also chemists.
While the goal of distillation is typically to extract ethanol, the real magic lies in shaping what else survives the journey through the still. Flavor-active compounds, volatile esters, heavier alcohols, and even sulfur compounds all play a role in defining a spirit’s character.
Thanks to the work of whisky flavour explorer John Angus, we now have a powerful framework to understand how four core elements—still shape, boiling point, reflux, and copper—act as filters to sculpt flavor during distillation.
Let’s explore how this hidden layer of chemistry determines what ends up in the glass.

A still’s design isn’t just aesthetic—it’s functional. Everything from neck height to lyne arm angle plays a role in how vapors behave.
Tall necks + upward lyne arms encourage reflux, which sends heavier vapors back down, refining the spirit.
Short necks + downward angles allow more heavy compounds through, resulting in a weightier, oilier distillate.
Wider necks or open-top stills increase contact with copper (more on that later), affecting sulfur and ester retention.
Your still shape is your first flavor decision.

Different compounds boil and vaporize at different temperatures. As the wash is heated, the lightest molecules rise first.
Heads (Foreshots) – Highly volatile compounds like acetaldehyde and methanol (20–80°C). These are usually discarded.
Hearts – Where the most desirable compounds live: ethanol, fruity esters like isoamyl and hexyl acetate (80–150°C).
Tails (Feints) – Higher boiling point alcohols like 1-hexanol and fatty acids like hexanoic acid (150–200°C+). These can add depth but often bring funk, sulfur, or bitterness.
The timing of your cuts defines the character of your final spirit—cut too early and you lose nuance; too late and the profile becomes heavy, even off-putting.

Reflux is the process where rising vapor condenses and falls back down, giving heavier elements another chance to be stripped out.
Still shape (as mentioned)
Cooling system efficiency
Collection rate (slow = more reflux)
Packing or trays (in column stills)
More reflux = cleaner, lighter spirit
Less reflux = oilier, richer spirit
Reflux is where balance begins. It gives distillers control over clarity versus complexity.

Copper isn’t just a historical material—it’s a functional filter.
Removes sulfur compounds like mercaptans, which cause off-notes (rubber, cabbage).
Enhances esters by allowing fruity, floral volatiles to shine.
Buffers acidity and pH by reacting with undesirable compounds.
Feeds esterification reactions, aiding the bonding of acids and alcohols into flavorful esters like ethyl acetate or isoamyl acetate.
The more surface area contact with copper, the brighter and smoother the spirit.
Want heavier flavor? Use stainless steel or reduce copper exposure. Want elegance and polish? Let copper do its job.
Each of these four filters is a lever. But they don’t work in isolation.
A taller still with lots of reflux might require later cut points. A short-necked pot still with minimal copper may need a slower run or tighter tails cut.
That’s the art of distilling—each decision influences the next.
As John Angus summarizes:
“It’s not just about collecting ethanol. It’s about shaping what survives the journey into the glass. Change just one variable, and the whole thing shifts.”
This visual breakdown and chemical insight are the work of John Angus, a leading voice in whisky education and flavor chemistry. His illustrations and commentary are used in training programs, distillery workshops, and academic settings worldwide.
Brindiamo is proud to feature John’s work with permission. If you’d like to explore licensing, visit his LinkedIn page here.

Why mature liquid remains scarce even as younger barrels soften — and what it means for buyers For the last two years, the dominant narrative in bulk...

As we reflect on 2025, we wanted to take a moment to look back with you. This past year, our team published across more mastheads than ever before —...
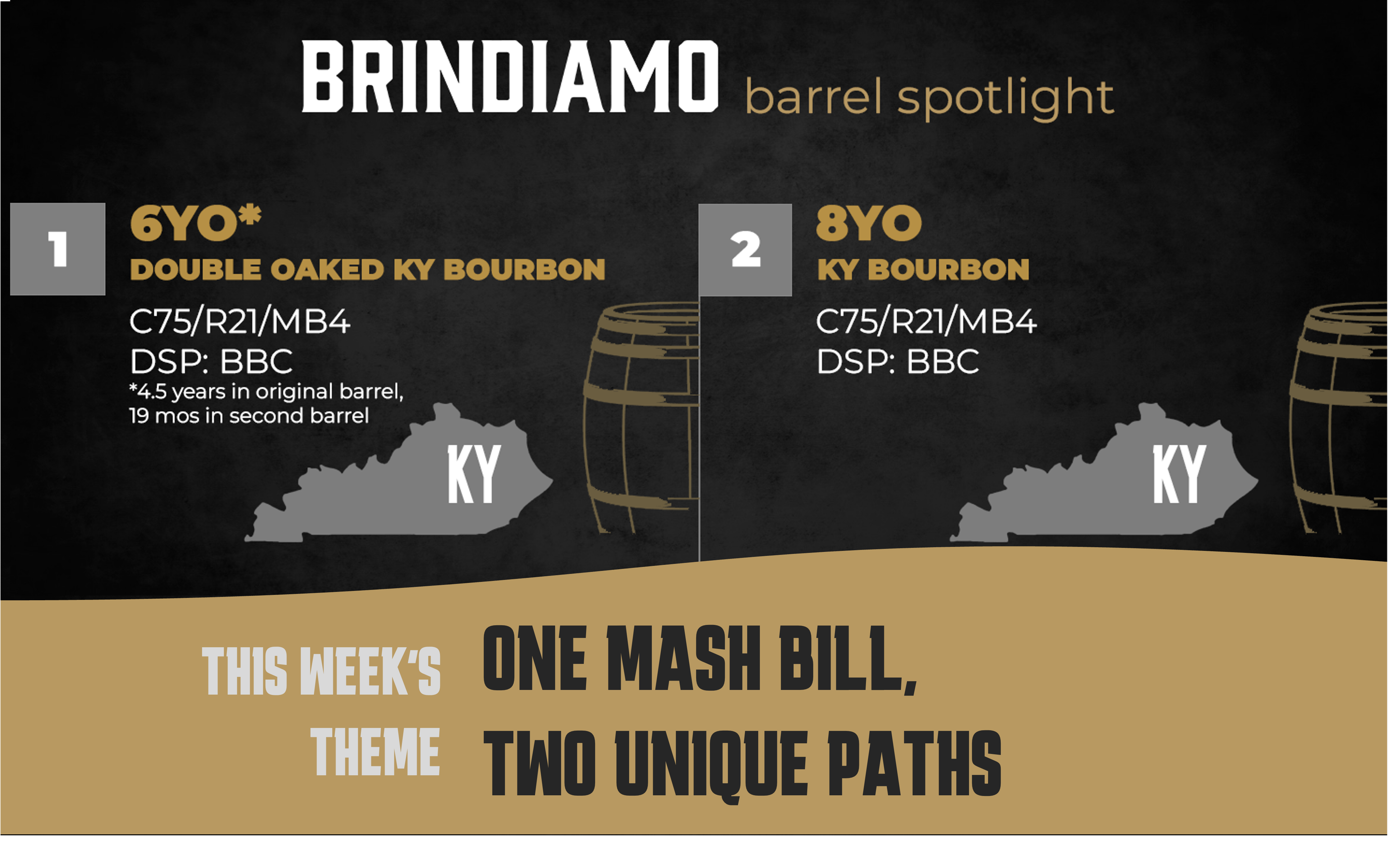
Welcome back to the Brindiamo Barrel Spotlight, our weekly email series highlighting the barrels, distilleries, and market dynamics shaping today’s...

Whiskey is one of the world’s most revered spirits, but not all whiskey is created equal. Two key categories—new fill whiskey and aged whiskey—play...

In the world of whiskey, age is more than just a number—it’s a testament to time, patience, and craftsmanship. As whiskey matures in oak barrels, it...

The global demand for whiskey continues to rise—and private labeling offers a powerful, profitable way for brands to enter or grow in the market....
Join the conversation
Leave a comment below.